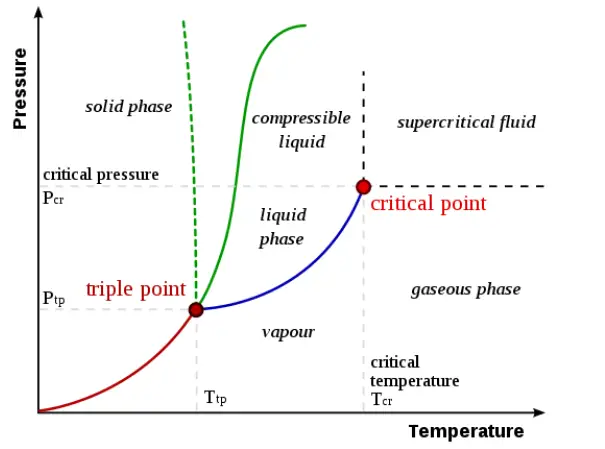
Der kritische Punkt, auch kritischer Zustand genannt, ist in der Thermodynamik ein Punkt in einem Phasendiagramm, an dem sowohl die flüssige als auch die gasförmige Phase eines Stoffes die gleiche Dichte aufweisen und daher nicht unterscheidbar sind.
Der Tripelpunkt eines Stoffes ist die Temperatur und der Druck, bei denen die drei Phasen (flüssig, gasförmig und fest) dieses Stoffes im thermodynamischen Gleichgewicht koexistieren.
Die wichtigsten Unterschiede
- Kritischer Punkt beschreibt die Koexistenz von zwei Phasen desselben Stoffes, während der Tripelpunkt die Koexistenz von drei Phasen desselben Stoffes beschreibt.
- The point at which saturated liquid and saturated vapor lines meet on a phase diagram is the critical point. On the other hand, the point at which fusion line and sublimation line and vaporization meet on a phase diagram is the triple point.
- For most of substances the critical temperature is usually higher than the standard temperature. On the other hand, the temperature corresponding to the triple point is usually lower than the standard temperature.
- At the critical point, only liquid and gaseous phases can coexist in equilibrium whereas at triple point, all solid, liquid and gaseous phases can coexist in equilibrium.
- Critical point is represented by a point on p-v-T surface. Triple point is represented by a point on p-T diagram but appears as a line on p-v-T surface.
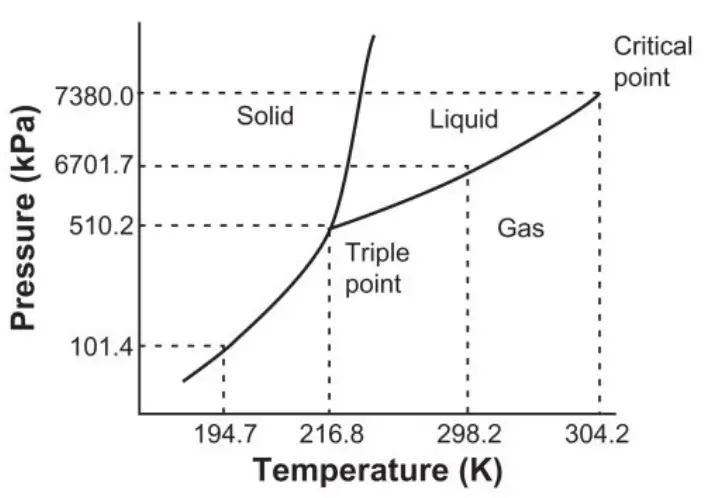
- An example of critical point is the liquid-vapor critical point, the end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist. An example of triple point is the triple point of mercury, the triple point of mercury occurs at a temperature of -38.83440 degrees Celsius and a pressure of 0.2 mPa.
- The critical point of water is at 647K and 22.064 mPa whereas the triple point of water is at 273.16 K and 0.6116557 mPa.
Critical point Vs. Triple Point In Tabular Form
| BASIS OF COMPARISON | CRITICAL POINT | TRIPPLE POINT |
| Description | Critical point describes the coexistence of two phases of the same substance. | Triple point describes the coexistence of three phases of the same substance. |
| Phase Diagram | The point at which saturated liquid and saturated vapor lines meet on a phase diagram is the critical point. | The point at which fusion line and sublimation line and vaporization meet on a phase diagram is the triple point. |
| Temperature | For most of substances the critical temperature is usually higher than the standard temperature. | The temperature corresponding to the triple point is usually lower than the standard temperature. |
| Coexistence At Equilibrium | At the critical point, only liquid and gaseous phases can coexist in equilibrium. | At triple point, all solid, liquid and gaseous phases can coexist in equilibrium. |
| Representation | Critical point is represented by a point on p-v-T surface. | Triple point is represented by a point on p-T diagram but appears as a line on p-v-T surface. |
| Example | An example of critical point is the liquid-vapor critical point, the end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist. | An example of triple point is the triple point of mercury, the triple point of mercury occurs at a temperature of -38.83440 degrees Celsius and a pressure of 0.2 mPa. |
| The Critical &Triple Point Of Water | The critical point of water is at 647K and 22.064 mPa. | The triple point of water is at 273.16 K and 0.6116557 mPa. |
